Abstract
Background: Allogeneic hematopoietic cell transplantation (alloHCT) is a life-saving procedure for the treatment of a variety of devastating diseases. The National Marrow Donor Program (NMDP) was founded in 1987 to facilitate alloHCT from anonymous volunteer unrelated donors. Hematopoietic stem cells are typically collected from unrelated adult donors in 2 ways: via unstimulated bone marrow (BM) harvest or via rHuG-CSF (filgrastim)-mobilized peripheral blood stem cell (PBSC) collection. For mobilization, donors are administered 10 µg/kg subcutaneous filgrastim daily for 5 days. Donor data collected to date have primarily focused on short-term donor adverse events (AEs), following both unstimulated BM and filgrastim-mobilized PBSC collection; hence, data on long-term AEs experienced by unrelated donors are limited. We hypothesized that volunteer donors receiving filgrastim would not be at increased risk of malignancy, autoimmune disorders, or thrombotic events compared with donors who did not receive filgrastim. To ascertain the incidence of long-term effects of filgrastim, the NMDP performed an observational study of unstimulated BM donors compared to filgrastim-mobilized PBSC donors.
Methods: The study consisted of a retrospective donor cohort comprised of donors who underwent a BM or PBSC collection between July 1, 1999, and September 30, 2010, and a prospective donor cohort comprising donors who underwent a collection after study activation on October 1, 2010. After donors provided informed consent, they completed biennial phone surveys to report malignancy, autoimmune, or thrombotic (MAT) events until study completion. When a MAT event was reported, the donor was asked for consent to obtain and review pertinent medical records, which were then confirmed by the NMDP medical services team. The study was closed to accrual on September 30, 2015, and follow-up phone call surveys were completed on September 30, 2020. Of the 32634 donors contacted, 21833 enrolled in the study. After screening for eligibility and removing those with missing or incomplete data, 21653 donors were included in the final analyses. P-values and 95% confidence intervals for the rate ratios were calculated using unadjusted Poisson regression methods.
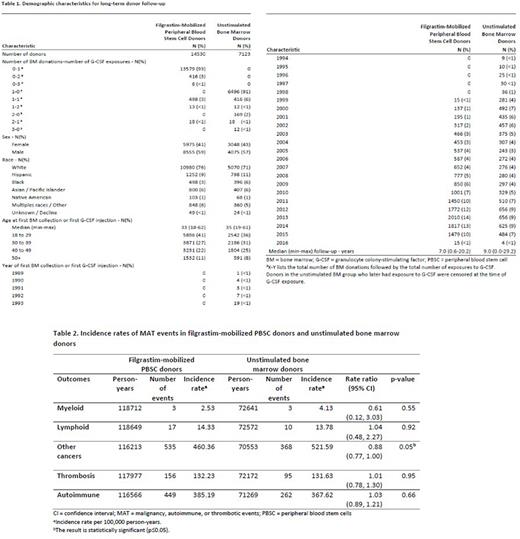
Results: Overall, 7123 donors were not exposed to filgrastim (BM donors) and 14530 donors were exposed to filgrastim (PBSC donors). Baseline donor demographics and clinical characteristics are shown in Table 1. The median time to last survey completion was 9 years (range 0-29.2) for BM donors and 7 years (range 0.6-20.2) for PBSC donors. Incidence rates and incidence rate ratios for myeloid malignancies (AML, MDS, CML, chronic myeloproliferative disorders), malignant non-myeloid hematologic disorders (ALL, CLL, Hodgkin lymphoma, non-Hodgkin lymphoma), non-hematologic malignancies, thrombotic events (venous and arterial), and autoimmune diseases (RA, psoriatic arthritis, SLE, scleroderma, vasculitides, MS, and immune thrombocytopenic purpura) are shown in Table 2. Overall, the incidence of MAT disorders in filgrastim-mobilized unrelated PBSC donors were not significantly different compared to unstimulated BM donors, supporting the hypothesis that donors who received filgrastim are not at higher risk of MAT events.
Conclusions: This is the largest and most comprehensive study of long-term effects of filgrastim on unrelated PBSC donors measuring outcomes related to MAT disorders. The results of this study provide strong evidence that donors who receive filgrastim are not at increased risk of these events compared to donors of BM and provide reassurance to current donors undergoing stem cell mobilization as well as to those considering signing up for stem cell registries such as Be The Match.
Disclosures
Stefanski:Novartis: Other: Ad Board, Speakers Bureau. Schenfeld:Amgen Inc: Current Employment, Current equity holder in private company. Sandschafer:Amgen Inc.: Current Employment, Current equity holder in publicly-traded company. Burns:Gamida Cell: Research Funding; Bristol Myers Squibb: Research Funding; Astellas Pharma Inc.: Research Funding. Shaw:OrcaBio: Consultancy; Mallinkrodt: Consultancy. Pulsipher:Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Sponsored Educational Talks; Gentibio: Membership on an entity's Board of Directors or advisory committees; Bluebird: Membership on an entity's Board of Directors or advisory committees; Vertex: Membership on an entity's Board of Directors or advisory committees; Medexus: Membership on an entity's Board of Directors or advisory committees; Equillium: Membership on an entity's Board of Directors or advisory committees; Miltenyi: Other: Sponsored Educational talks & Study Support; Adaptive: Other: Study support. Devine:Orca Bio: Consultancy, Other: Payment to NMDP for consulting.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal